Merkel cell carcinoma (MCC) is not a common skin cancer, but it is a very dangerous one. The number of cases has increased dramatically in the past decade. Luckily, knowledge and treatment options have surged, too. Paul Nghiem, MD, PhD, director of the Skin Oncology Clinical Program at the Seattle Cancer Care Alliance and head of Dermatology at the University of Washington School of Medicine, among other titles, has worked tirelessly to promote research and collaboration to create better outcomes for MCC patients who previously had little hope.
 With so many advances on the horizon, we thought this was a good time to raise awareness among dermatologists and other medical professionals about MCC in our current issue of Carcinomas & Keratoses. With his well-known energy and enthusiasm, Dr. Nghiem engaged in a lively conversation with Julie Bain, The Skin Cancer Foundation’s senior director of science & education, and Désirée Ratner, MD, our C&K editor-in-chief, to share his expertise and shed light on the most important advancements in his field.
With so many advances on the horizon, we thought this was a good time to raise awareness among dermatologists and other medical professionals about MCC in our current issue of Carcinomas & Keratoses. With his well-known energy and enthusiasm, Dr. Nghiem engaged in a lively conversation with Julie Bain, The Skin Cancer Foundation’s senior director of science & education, and Désirée Ratner, MD, our C&K editor-in-chief, to share his expertise and shed light on the most important advancements in his field.
Hot Topics in the Care of Patients with Merkel Cell Carcinoma (MCC)
Julie Bain: Dr. Nghiem, you helped plan the first-ever International Symposium on Merkel cell carcinoma (MCC) in Tampa in October 2019, promoting more collaboration in the field. What was not happening that collaboration could help solve?
Paul Nghiem, MD: Everything can be accelerated by more collaboration! Although many attendees knew each other and had been interacting for years, having a meeting focused solely on MCC — the first in which abstracts were sent in and judged — was exciting for our field and brought it to a different level.
With a rare cancer, you need multi-institutional collaboration to have the expertise, the samples, the patients and the data to be as impactful as possible. The meeting proved to some of our pharmaceutical partners that there’s a lot of scientific interest and patient interest. We’re already planning the next one.
JB: Some quick basics: What are Merkel cells, and how are they involved in MCC?
PN: People wondered for over a century what Merkel cells do, and that answer was provided around 2009 when studies clearly showed that they are touch sensors. So German physician Friedrich Merkel was correct in 1875 when he called them touch cells. MCC got its name because it looks like normal Merkel cells in the skin. But now we believe that MCC doesn’t actually derive from normal, mature Merkel cells.
JB: What are the origins of this rare cancer?
PN: About 80 percent of MCCs are associated with MCC polyomavirus, but there’s a discrepancy of thought about the source of these virus-positive Merkels. Some experts think they arise from epidermal cells. Others think they come from dermal fibroblasts. A few people think they may come from B lymphocytes.
MCCs that are not caused by the virus are entirely caused by ultraviolet (UV) radiation. They’re heavily UV-mutated. We’re quite confident that there was never another virus there. With virus-negative MCC, we pretty much know that it comes from keratinocytes. So yes, at least that type is a keratinocyte carcinoma.
We don’t yet understand the role of UV in virus-positive MCCs. They seem to be more prevalent on sun-exposed skin and in fair-skinned individuals, so there’s probably some UV cofactor, but they’re not heavily UV-mutated.
JB: At The Skin Cancer Foundation, early detection is an important part of our messaging. Is there a way to identify MCC at its earliest stage?
PN: Unfortunately, MCC tumors look very unconcerning until they are advanced, and the only way to make the diagnosis is under the microscope. The biggest lesson to learn is that when the surprise diagnosis of MCC comes back in what you thought was a cyst, that’s when you need to do your homework about getting the right kind of care for this cancer.
With about 3,000 MCCs diagnosed in the U.S. each year, there’s not really a public health message, except for patients with many risk factors for skin cancers in general, including lots of sun exposure, prior skin cancers, immune suppression. Those people need to be watched very carefully because they could have a serious skin cancer that might look pretty boring. A red, firm bump on a person at risk for skin cancer might deserve a biopsy, even though it might not look like a classic skin cancer.
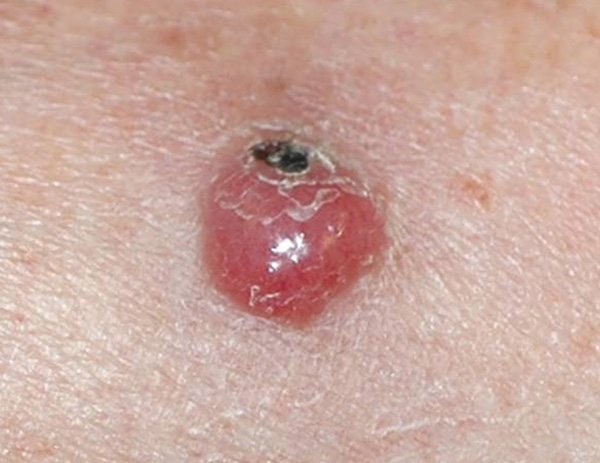
A recurrence of Merkel cell carcinoma on the forehead.
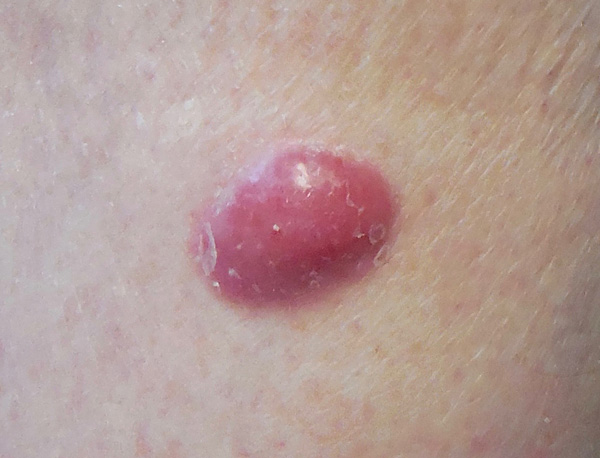
Merkel cell carcinoma on the lower leg.
JB: What do you advise about timing from diagnosis to treatment?
PN: When the diagnosis of MCC comes up, most doctors and patients quickly realize this is something to take seriously. MCC is aggressive and dangerous, and we certainly like to get people into evaluation, scans and treatment within, say, two weeks — to get a good plan and act on it. But it is important not to rush; get the facts before you jump.
JB: How important is it to consider multidisciplinary care at a cancer center for MCC?
PN: It’s so important, for several reasons. Depending on your sources of data, MCC is three to five times more likely to kill a patient than melanoma, so it’s a serious diagnosis. And, due to its rarity, a lot of physicians are not familiar with the specific care that’s indicated. Frankly, updated information is not in many textbooks because MCC management has been so rapidly evolving over the past five to 10 years. Going to a place that truly offers expert multidisciplinary care is very important.
Another reason is that, unlike typical early melanoma, which is mostly a surgical disease, MCC often also needs radiation or at least discussion of radiation, and before long it’s not only surgery and radiation and dermatology involved, but also medical oncology for adjuvant treatment and/or distant disease.
Désirée Ratner, MD: With MCC, from the beginning, you have to think about it as comparable to an aggressive melanoma or a high-risk squamous cell carcinoma, so you’re anticipating disease going beyond the skin very quickly. I can manage a melanoma easily as long as it’s under 0.8 millimeters, so it doesn’t require a sentinel lymph node biopsy.
But multidisciplinary care needs to happen quickly when you have a deeper or more aggressive tumor. Most other skin cancers stay localized for a long time and you have more latitude in terms of time to get them treated appropriately. You don’t have that with MCC, hence the need for multidisciplinary care so much sooner.
PN: A perfect point, because multiple studies have shown that even the smallest MCCs, down to just a few millimeters, have at least a 10 or 15 percent chance of already having spread to lymph nodes, going up to 40 or 50 percent from there.
JB: Is there a clear difference in prognosis between virus-positive and virus-negative MCC?
PN: The virus-negatives in general are more aggressive. They’re about 30 to 40 percent more likely to come back, everything else being the same, than virus-positives, and tend to recur more aggressively, both locally and distantly.
JB: Is there a role for Mohs surgery in MCC treatment?
DR: As a Mohs surgeon, I don’t do Mohs for MCC anymore because I believe that sentinel node biopsies and wide excisions need to be done contemporaneously. However, if you tell me that there’s a reason to implement Mohs surgery and tissue sparing for MCC, then I’ll think about it.
PN: Bingo. I totally agree. It’s mostly extra hassle for the surgeon and the patient to separate the sentinel node biopsy from the excision. Now, if you’re not going to do the sentinel node biopsy, that’s a different situation, but usually a sentinel node biopsy should at least be considered. There’s also the issue of radiation. If you’re going to radiate, you don’t need wide margins. You don’t even need microscopically negative margins if you’re going to radiate.
Mohs surgeons know skin cancer better than anybody, so they’re in a great position to be the orchestrator in managing MCC patients, since they can refer them to their colleagues in radiation, medical oncology and surgical oncology. As a dermatologist, I don’t do the big surgery, I don’t do chemo and I don’t radiate. But I help the patient navigate their care optimally among those disciplines.
DR: I think it may be helpful to step back for a minute to understand your current approach to the MCC patient. Your standard approach would be surgery with consideration of a sentinel lymph node biopsy. If the sentinel node is negative, you wouldn’t radiate the node bed. If the sentinel node is positive, would you then radiate the primary site and the draining nodes, or would you do a completion dissection?
PN: For sentinel-node positive disease, we typically radiate the primary site and the involved node bed and do not perform a completion node dissection. By definition, a sentinel node means there was no clinically evident nodal disease, so there’s not such a heavy burden in the lymph nodes.
DR: So if there are clinically positive nodes — if the patient comes in right off the bat with new primary disease and nodal metastases — how do you stratify your thinking?
PN: Well, it depends on the morbidity of the surgery. We almost always try to get the gross disease out surgically even if we’re planning on doing radiation to eliminate the microscopic disease. Recently though, you’ve got this extra consideration of immunotherapy. If the tumor is not operable, it gets simpler. You’re going to do some kind of systemic immune therapy. But if it’s operable with a lot of morbidity and you’re expecting an 80 percent recurrence rate after that, then it’s a tougher decision and you might start thinking about immunotherapy more up front.
JB: What are the data on recurrence rates after surgery versus surgery plus radiation?
PN: Some of the top cancer centers have fantastic surgeons, whose data show lower MCC recurrence rates, in the absence of radiation, than other centers, including in the literature. One challenge regarding the published data on recurrence after surgery is that it does not always separate out patients who received local adjuvant radiation from those who did not. When you radiate your highest-risk patients, you’re stopping the cancer from coming back, since radiation is very effective for local control. This makes it difficult to interpret recurrence risks after surgery.
We have a paper that we’ve been working on for several years and recently submitted that I think helps bring this field together. You can’t determine the right surgical margin if you’ve lumped together tumors that have and have not received local radiation — because with local radiation you get great control, whatever your margins were. And if you don’t radiate MCC, you really need margins greater than 1 centimeter to get good control.
If you’ve got a low-risk lesion and you’re able to get wide margins that are not mutilating and the patient is not immunosuppressed and has negative sentinel lymph nodes, then you’re probably OK with surgery alone. But with more and more risk factors, you raise the risk of local recurrence if you don’t give some radiation.
DR: What is the dividing line between a lower-risk and a higher-risk tumor?
PN: To be lower-risk, that’s a pretty simple list: Number one, ideally it should be less than 1 centimeter, but maybe less than 2 centimeters as a primary size. Number two, ideally it would be sentinel node-negative. Number three, it would have pathologically negative surgical margins. Number four, the patient would not be profoundly immunosuppressed. Number five, there would not be lymphovascular invasion. And number six, it would not be on the head and neck, which, by itself, has a higher rate of recurrence. There are multiple studies showing increased risks with these factors. We’ve certainly seen that in Seattle, where we’ve had a 15 to 20 percent local recurrence rate for non-irradiated MCCs on the head and neck, even if all of the other factors were negative.
JB: If the tumor doesn’t meet those criteria and is at higher risk, what do you recommend?
PN: I think it’s relevant to mention that we used to have only two choices with radiation: zero radiation, or about 50 Gy over six weeks. However, now we’re doing just one dose of radiation with 8 Gy, which shrinks advanced MCC tumors very nicely. We’re now studying this radiation approach in the adjuvant setting. There are lots of patients who don’t want six weeks of radiation with nasty side effects, but we know there is appreciable risk with giving them no radiation. This is like Goldilocks, where there’s something in the middle that may be just right. Our single dose 8 Gy regimen has essentially no side effects, and if there were a gross tumor there, it would get rid of it in 94 percent of cases. Data are early in this adjuvant setting, but it seems to be working about as well as the six-week treatment, with much fewer side effects.
JB: Let’s talk a bit about immunotherapy and what looks most promising for MCC.
PN: I think it’s safe to say that immunotherapy — meaning avelumab, pembrolizumab or retifanlimab — is a much better approach to treating advanced MCC than chemotherapy. And the trick there is that their initial response rates are essentially the same. About 60 percent of patients’ tumors will shrink from either chemotherapy or immunotherapy, but the chance of staying in response once you’ve entered it is very different. Only 5 percent of chemotherapy responders will have their response persist for a year, while 80 percent of immunotherapy responders will have their response persist for over a year.
The side effects are also different. The side effects for chemotherapy, at least initially, are not too bad, but it later really decreases quality of life and suppresses immune function. Immunotherapy side effects are highly unpredictable, with about half of patients just having some fatigue, and a third of patients having significant autoimmune side effects.
JB: Combinations are being studied?
PN: Yes, but there’s not yet good data that one could quote. The only major combination is ipilimumab and nivolumab, and the Bristol-Myers Squibb trial looking at that in MCC has not been reported yet, so we just don’t know.
JB: And similarly, immunotherapy plus radiation?
PN: It’s not routinely done, and I think that’s appropriate. Up front, you want to make a choice and give one agent. I’m a big fan of radiation, but when one agent like immunotherapy is going to work in 60 percent of cases, we shouldn’t do it for no reason. So, we reserve radiation until, let’s say, two of the tumors shrink, one doesn’t, and then we might radiate the remaining one to encourage it to go away.
JB: What about immune-related side effects?
PN: Multiple clinical trials suggest that people with immune-related adverse events, who start attacking other parts of their body with their immune system, actually survive their cancer better.
The best example is melanoma, where those side effects are protective and associated with better survival. Having autoimmune reactions actually predicts better control of your cancer. So we think this side effect has a sort of silver lining indicating that the immune system is getting more revved up by the drug and also doing a better job fighting the cancer.
JB: What’s new in neoadjuvant therapy?
PN: There is an ASCO presentation from 2018 of a clinical trial of about 39 patients, which formally looked at neoadjuvant nivolumab, two doses before surgery, with about a 60 percent major response. That means either complete tumor elimination or shrinkage such that less than 10 percent of viable tumor cells are left after four weeks.
That does mean, though, that we’ve got 40 percent of patients who didn’t respond well or at all. There are many nuances in this area, and it’s philosophically challenging because some patients are not going to benefit, and their care will be delayed. Some may have side effects with no benefit, while other patients’ cancers will be gone. But we have no way of predicting who’s going to respond fantastically and who’s not.
The most useful predictor of all, though, is if you end up giving a little bit of immunotherapy before surgery, patients’ initial response or lack of response is quite predictive of their subsequent disease control.
JB: How do you use the AMERK biomarker blood test?
PN: We believe in getting one assessment at baseline to figure out whether the patient makes antibodies to Merkel cell polyomavirus. About half of MCC patients make those antibodies and half do not.
JB: What information does this test give you?
PN: It doesn’t tell you which kind of treatment you need. It’s more for screening MCC patients and helping recurrent disease to be caught earlier. For patients who don’t make antibodies, this will never be a useful test and they don’t need to have it done in an ongoing way. They have a 40 percent higher risk of their cancer coming back, so they need to be followed closely with scans and exams.
In contrast, the patients who do make antibodies probably need fewer scans, certainly once their antibody titer falls. This test is more sensitive and specific than scans and spares those patients the IV contrast, the cost and the radiation of the scan. For patients whose antibodies go down and stay down, we don’t have one example among hundreds of these patients of the cancer coming back. They don’t need scans. When their antibodies go up, they get a scan. It’s a really good test.
JB: Any new therapies coming down the road that look promising?
PN: Quite a few intralesional immune stimulating agents are being explored. One that isn’t extensively being studied in MCC, but this audience will know pretty well, is T-VEC, or Imlygic, an oncolytic virotherapy that does seem to have some efficacy in MCC. Several other agents are being explored as well. All of them turn on the innate immune system, which is the ancient one that leads to damage signals and helps the lymphocytes or the adaptive immune system to work better. I think that another exciting direction is a therapeutic vaccine, which is at least being considered for development — as well as immunotherapy in the adjuvant and neoadjuvant setting.
JB: Would those intralesional therapies help with checkpoint blockade therapies in a synergistic way?
PN: Absolutely. All of those would be combined with immune checkpoint therapy unless it were super-early disease. You could imagine a neoadjuvant procedure where for a week or two before surgery you do something to boost an anti-tumor immune response before excision of the tumor. If that were effective, it would be much safer than giving the whole body anti-PD-1. That way you get the immune response properly on track in the tumor and hope to get some effective T cells going around through the body.
JB: Isn’t it amazing how much has happened in just a decade?
PN: Yes, it’s mind-boggling. I was running around only a little more than 10 years ago saying there’s no way there’s a virus causing MCC.
JB: You probably thought there was no way you would cure one, either.
PN: Exactly. I mean we knew we had good chances for early disease with surgery and radiation but, my gosh, with systemic disease I just thought, “No, that’s hopeless.”
JB: How would you compare now to then, as far as patients with metastatic disease being potentially cured?
PN: That’s a provocative question. With 60 percent of patients initially responding and the majority of those remaining in response for at least a couple of years, and the majority of those then stopping immunotherapy and not having it come back, that’s an enormous improvement. We’re left with around a third that go off therapy long-term without any evidence of their cancer coming back.
Some of those are probably going to recur, but I’m confident not all of them will. I think we are effectively curing at least some patients. Can we prove there’s not one cancer cell left in a given patient? Of course not. But I think we can now say with confidence that many patients with advanced disease are going to have something other than MCC take them to the next level of celestial existence.
JB: What are you intensely focusing on next?
PN: We’re really, really focused on refractory disease, so PD-1 nonresponders. I don’t think we’ll suddenly be able to predict who’s going to respond and who’s not. Thousands of scientists in every kind of cancer are trying to figure that out, and it’s not been easy going. I am optimistic, though, that we’re going to make progress with PD-1 refractory MCC patients. We see a lot of patients whose cancers come back after PD-1, responding to various treatment combinations. I’m confident that we’ll get better at managing those patients.
Personally, I’m super excited about the idea of keeping the immune drug on and adding a low dose of radiation in the setting of a radiation sensitizing drug. Those are just becoming available. We’re talking about the next generation of DNA damage response inhibitors, which selectively kill rapidly dividing cancer cells given small amounts of radiation. The fascinating thing is that these new drugs kill cancer cells in a way that turns on an immune response.
We can give low doses of radiation, as I said earlier, and 8 Gy will get rid of 90 percent of MCC tumors. One dose. We can lower the dosage even more, and then give one of these drugs that selectively kills cancer cells after they’ve been dividing like crazy and they’ve gotten a little bit of DNA damage, which makes them immunogenic as they die. How cool is that?
So that’s where my dream is right now. I started to work in that DNA damage response area when I was a postdoctoral fellow and have continued to be involved. We thought it was really cool, but we didn’t have the drugs. It took many years for the pharmaceutical companies to come up with the DNA damage response inhibiting drugs that now exist. They’re in phase 1 and 2 trials, and it’s the perfect time to combine that with a little bit of radiation for patients with refractory disease. That’s what I’m really excited about.

Désirée Ratner, MD
Editor-in-Chief, Carcinomas & Keratoses
How Far We’ve Come in MCC Treatment!
I first learned about Merkel cell carcinoma (MCC) as a medical student, when an elderly man came in for Mohs surgical follow-up. My attending explained that this patient had had a highly aggressive tumor, Merkel cell carcinoma, which might as well be called “Murky cell carcinoma” because it was so poorly understood. My interest was piqued, and that spurred me as a resident to write a review article on the subject (now, of course, out of date). MCC was unusual among skin cancers in that it was so difficult to diagnose and its behavior was so aggressive, with a large number of patients developing metastases and succumbing to their disease within just a few years.
I began seeing MCC patients of my own in the late 1990s. There weren’t yet any guidelines for management of this tumor, and the common denominator for most of my patients was that they died of their disease. Wide local excision with sentinel lymph node biopsy (SLNB) was recommended for trunk and extremity tumors, so I sent those patients to surgical oncology. Even with negative SLNBs, patients with small, “early” tumors still developed metastases. Patients with positive SLNBs received adjuvant radiation and sometimes underwent complete lymph node dissection, but it was impossible to predict who would survive. Head and neck MCCs were treated with Mohs surgery, followed by radiation to the site and the draining nodes if the tumor was difficult to clear. My longest surviving patient received adjuvant radiation and chemotherapy with cisplatin and etoposide after his Mohs slides revealed MCC in his parotid. He moved to North Carolina two years after treatment, in good health, and stopped by to visit me a year later, still alive and well. He was the fortunate exception.
While only about 3,000 MCC cases are diagnosed in the U.S. each year, the numbers have been increasing. Discovery of the Merkel cell polyomavirus, and recognizing the importance of initiating early and aggressive treatment for MCC patients, has, however, made us better at managing their disease than we used to be. Since 2010, the National Comprehensive Cancer Network has published MCC guidelines that are regularly updated by a multidisciplinary panel and include algorithms for workup and management. There is now an updated AJCC (8th edition) staging classification categorizing MCC patients based on their primary tumor size as well as their clinical and pathological extent of disease. While surgery and radiation are more predictably effective for patients with lower risk disease, three immunotherapeutic agents, avelumab, pembrolizumab and, in 2023, retifanlimab-dlwr, have been FDA approved for advanced MCC, giving patients whose short-term prognosis was once quite grim increased hope of extended disease-free survival.
This issue of C&K features an interview with Paul Nghiem, MD, PhD, who helped lead the clinical trials that led to these FDA approvals and recently co-organized the first-ever international MCC symposium. Dr. Nghiem told us that, “with a rare cancer, you need multi-institutional collaboration to have the expertise and the samples and the patients and the data to be as impactful as possible.” His international symposium highlighted new research and clinical innovations in the field of MCC, but also demonstrated to patients and the health-care community the level of scientific interest in and dedication to MCC that currently exists. With the significant progress that Dr. Nghiem and his colleagues have made in a relatively short time, who knows? They might succeed in solving even the murkiest problems posed by this unusual tumor and, in so doing, create a totally new paradigm for multidisciplinary cancer care.
See SkinCancer.org for patient-focused information on Merkel cell carcinoma: https://www.skincancer.org/skin-cancer-information/merkel-cell-carcinoma/
Visit merkelcell.org, in which Dr. Nghiem and other physicians and researchers who work on MCC have assembled resources to answer frequently asked questions about the disease: https://merkelcell.org/
Read a good, brief, recent summary of issues in MCC treatment in this article, “Less Toxic, More Effective Treatment — A Win-Win for Patients with Merkel Cell Carcinoma”: https://jamanetwork.com/journals/jamadermatology/article-abstract/2749357